Application of the PS affinity method to ELISA
We developed PS Capture™ Exosome ELISA Kit by applying affinity binding of Tim4 protein with exosomes. This kit is capable of detecting exosomes at a sensitivity higher than that of conventional ELISA methods immobilization of antibodies against exosome surface markers. Exosomes in samples such as culture supernatants and serum are captured by Tim4 protein on adry plate in the presence of calcium ion. The captured exosomes are detected by a primary antibody against an exosome surface marker protein and a labeled secondary antibody. While a mouse anti-CD63 monoclonal antibody is provided with the kit, a user-provided mouse primary antibody against any other exosome surface marker may also be used for exosome detection.
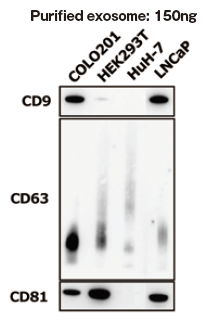
The greatest feature of this kit is that it provides exosomes detection with higher sensitivity than that of Western Blot analysis and conventional product for exosome ELISA. First, the detection limit for exosomes in Western Blotting was examined for comparison with this kit (Figures 1a and b). Western Blot analysis of exosomes purified from COLO201 cells (of human colon adenocarcinoma origin) with an anti-CD63 monoclonal antibody detected exosomes in an amount as small as 75 ng on the protein basis. Next, the detection limits of this kit for exosomes purified from K562 cells (of human leukemia origin) and COLO201 cells were determined to be 49.9 pg and 10.9 pg, respectively, demonstrating that this kit had a detection sensitivity more than 1,000 times higher than that of Western Blotting (Figure 1c). Considering that the detection limits of conventional products for exosome ELISA range approximately several ng to several μg (refer to instruction manuals for individual products), the present results demonstrated that this kit utilizing affinity binding of exosomes to Tim4 via PS has a sensitivity more than 100 times higher than those of conventional ELISA methods involving immobilization of an antibody against an exosome surface protein marker.
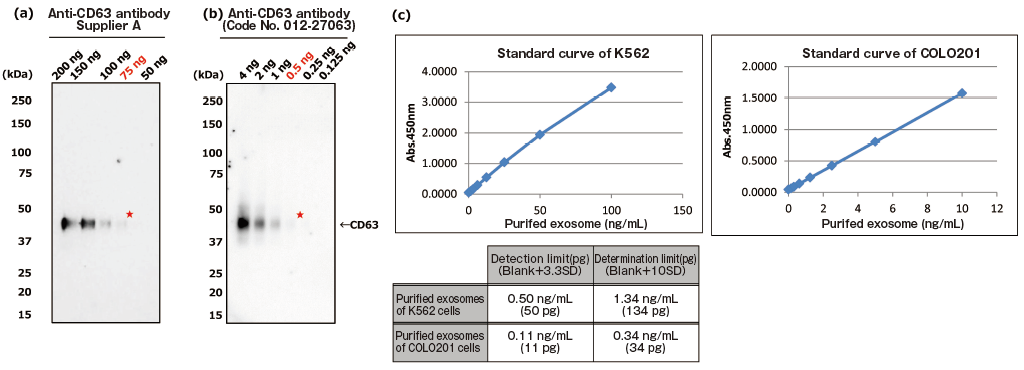
PS ELISA detected the marker proteins with 50-1,000 times higher sensitivity than WB.
| Fig. 1 Comparing the detection sensitivities of Western Blot and PS ELISA | ||
| (a), (b) Result of sensitivity by Western Blot with each of anti-CD63 antibody (supplier A and Wako: Code No. 012-27063). Sample: purified extracellular vesicles from cell culture supernatant of COLO201 cells with MagCaptureTM Exosome Isolation Kit PS (Code No. 293-77601) |
||
| ★:detection limit by Western Blot | ||
| (c) Result of limit by PS ELISA | ||
| A standard curve was prepared using blank value of buffer and absorbance value of 2-fold serial dilution samples of extracellular vesicles purified from cell culture supernatant of K562 cells and that of COLO201 cells with MagCapture™ Exosome Isolation Kit PS. Then, the detection limit of purified extracellular vesicles of K562 cells and COLO201 cells were calculated using its standard curve. (each dilution point: n=6, blank: n=12) | ||
PS Capture™ Exosome ELISA Kit(Anti Mouse IgG POD)
Features
- High sensitivity (detectable at a sensitivity 50-1,000 times higher than that of WB)
- Direct qualitative/quantitative analysis of exosomes in the culture supernatant
- Capable of saving the number of exosomes used for analysis (less than 1/10-1/1,000 of the number required for WB)
Application Data
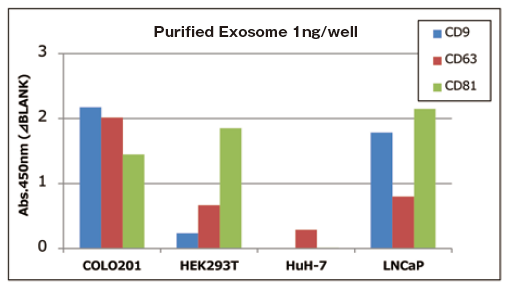
Qualitative analysis of extracellular vesicles purified from various cell culture supernatants
Add 1 ng of extracellular vesicles purified from various cell culture supernatants to each well, and the expression level of surface marker proteins was compared by a qualitative analysis with three primary detection antibodies.
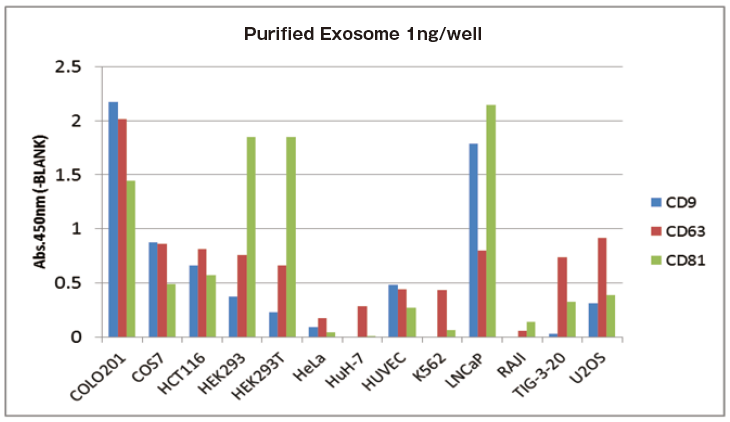
In addition, as reference comparative data, 150 ng of extracellular vesicles were purified from various cell culture supernatants and expression levels of their each surface markers were detected by Western Blot similarly. Then, qualitative analysis was conducted.
-
Comparison of qualitative data per 1 ng of purified exosomes
-
Reference comparative data

Expression pattern of marker proteins between ELISA and WB have a correlation.
Reference data: Qualitative analysis of EVs purified from various cell culture supernatants
Detection antibody
ELISA
Anti-CD9 mouse mAb (M-L 13), BD Bioscience
Anti-CD63 mouse mAb (H5C6), BD Bioscience
Anti-CD81 mouse mAb (JS-81), BD Bioscience
WB
Anti-CD9 rabbit pAb, System Bioscience
Anti-CD63 mouse mAb (8A12), CosmoBio
Anti-CD81 mouse mAb (1D6), Novus Biologicals
With 1 ng of extracellular vesicles purified from various cell culture supernatants to each well, expression level of surface marker proteins were detected by using three primary detection antibodies for CD9, CD63, and CD81.
It is confirmed that expression levels of particular markers on exosomes is different between cell strains.
Qualitative analysis of extracellular vesicles purified from human normal serum
Each of 40, 20, and 10 ng of extracellular vesicles purified from six human normal serum samples was added to a well and qualitative analysis was conducted using a control primary detection antibody against CD63 in the Kit.
Comparison of qualitative data of each sample
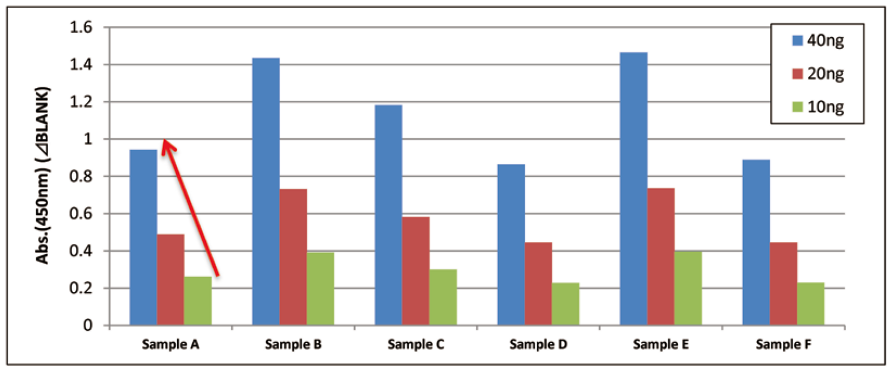
The results showed properly linear curves in each samples.
Reference data: dilution linearity of cell culture supernatant sample
A standard curve was prepared using extracellular vesicles purified from cell culture supernatant of COLO201 cells, and then the dilution linearity of 5-step dilution samples of cell culture supernatant of COLO201 cells (1:100 to 1:1600) was evaluated.
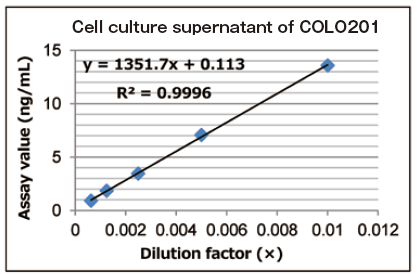
| Cell culture supernatant of COLO201 cells | |||||
|---|---|---|---|---|---|
| CM volume (µL) | Dilution | Assay value | Expected value | % of expected | |
| Ratio | Factor (x) | ng/mL | ng/mL | ||
| 0.0625 | 1 : 1600 | 0.000625 | 0.89 | 0.91 | 98.4 |
| 0.125 | 1 : 800 | 0.00125 | 1.82 | 1.72 | 105.6 |
| 0.25 | 1 : 400 | 0.0025 | 3.44 | 3.52 | 97.8 |
| 0.5 | 1 : 200 | 0.005 | 7.04 | 6.78 | 103.9 |
| 1 | 1 : 100 | 0.01 | 13.6 | - | - |
Reference standard: extracellular vesicles purified from cell culture supernatant of COLO201 cells with MagCapture™ Exosome Isolation Kit PS
Measured sample: cell culture supernatant of COLO201 cells
Primary antibody: anti-CD63 antibody in the kit
The results showed properly linear curves in each samples.
Monitoring changes of the amount of extracellular vesicles over time by the number of seeding cell and culture day
1 x 107, 2 x 107, and 3 x 107 COLO201 cells were separately seeded into T75 flasks and cultured for 72 hours. The small amount of culture supernatant samples were collected every 24 hours and subjected to spectrophotometric assay of CD63 using PS Capture™ Exosome ELISA Kit (Anti Mouse IgG POD) and Control Primary Antibody Anti-CD63 (100 x ) included in the kit.
For the CD63 assay, 4 μL cell culture supernatant was diluted to 100 μL with Reaction/Washing Buffer (1×) supplemented with Exosome Binding Enhancer.
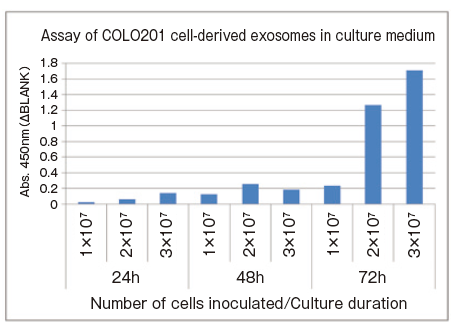
- Assay sample: 25-fold diluted COLO201 cell culture supernatant (4μL → diluted to 100μL)
- Number of seeding cells: 1 x 107, 2 x 107, 3 x 107 cells/T75 flask
- Culture duration: 24, 48, 72 hours
- Primary antibody: anti-CD63 antibody
Since it is possible to directly measure the amount of extracellular vesicles in the medium using only 4 μL of medium, this assay is recommended because an optimization of culture condition for newly culturing a cell line takes time and effort. By this method, the conditions under which the most extracellular vesicles are secreted can be easily examind.
Changes in extracellular vesicle production induced by addition of chemical agent
K562 cells were seeded into T225 flasks and cultured in serum-free medium for 72 hours. Then, the culture medium was changed to serum-free medium supplemented either with or without monensin sodium salt whose final concentration is 10 μM and cultured for 24 hours. After the end of culture, culture supernatant samples were collected and subjected to spectrophotometric assay of CD63 using PS Capture™ Exosome ELISA Kit (Anti Mouse IgG POD) and Control Primary Antibody Anti-CD63 (100 x ) included in the kit.
10-fold and 100-fold diluted cell culture supernatant samples were prepared by dilution of collected cell culture supernatant with Reaction/Washing Buffer (1 x ) supplemented with Exosome Binding Enhancer.
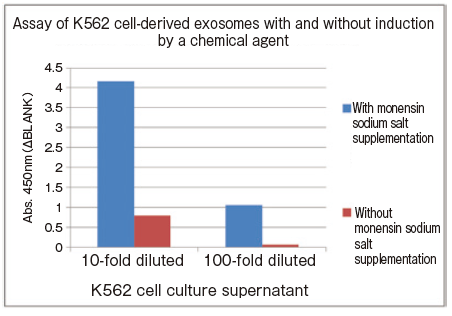
- Assay sample: K562 cell culture supernatant (cultured for 24 hours after changing to culture medium supplemented with monensin sodium salt or control culture medium)
- Primary antibody: anti-CD63 antibody
This assay requires no sample purification and just a small aliquot of culture medium collected during culture is sufficient for assay.
Since changes of the amount of exosomes in culture medium over time can be assayed and quantified, comparative assay of them is much easier than WB analysis. It's very convenient.
Comparison of detection sensitivity of various ELISA kits using exosomes purified from COLO201 cell culture supernatant and human serum
The following samples (1) to (6) were prepared and used for comparison of detection sensitivity of PS Capture™ Exosome ELISA Kit, Competitor A ELISA kit, and Competitor A ELISA kit (high-sensitivity type) with detection of CD63, an exosome marker protein.
Samples used for comparison
(1) Standard included in Competitor A ELISA kit
(2) Standard included in Competitor A ELISA kit (high-sensitivity type)
(3) Exosomes purified from COLO201 cell culture supernatant using MagCapture™ Exosome Isolation Kit PS
(4) Exosomes purified from COLO201 cell culture supernatant by polymer precipitation
(5) Exosomes purified from human serum using MagCapture™ Exosome Isolation Kit PS
(6) Exosomes purified from human serum by polymer precipitation
Dilution rates and protein concentrations
| (1) | (2) | (3) | (4) | (5) | (6) | |
|---|---|---|---|---|---|---|
| x 1 | 1/16 | 1/1000 | 40 ng/mL | 160 ng/mL | 800 ng/mL | 2000 µg/mL |
| x 0.5 | 1/32 | 1/2000 | 20 ng/mL | 80 ng/mL | 400 ng/mL | 1000 µg/mL |
| x 0.25 | 1/64 | 1/4000 | 10 ng/mL | 40 ng/mL | 200 ng/mL | 500 µg/mL |
| x 0.125 | 1/128 | 1/8000 | 5 ng/mL | 20 ng/mL | 100 ng/mL | 250 µg/mL |
| x 0.0625 | 1/256 | 1/16000 | 2.5 ng/mL | 10 ng/mL | 50 ng/mL | 125 µg/mL |
| 0 (BLANK) | 0 | 0 | 0 | 0 | 0 | 0 |
Comparison of detection sensitivity of individual exosome ELISA kits
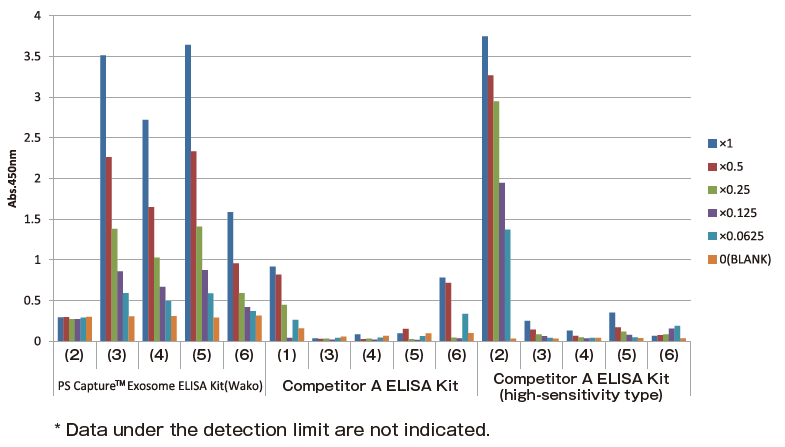
PS Capture™ Exosome ELISA Kit detected CD63 at a sensitivity higher than those of Competitor A ELISA Kit and Competitor A ELISA Kit (high-sensitivity type). While both Competitor A ELISA Kit and Competitor A ELISA Kit (high-sensitivity type) strongly reacted with standards in their kit, their reactivity to exosomes purified by the PS affinity method was low.
These results suggested that PS Capture™ Exosome ELISA Kit was capable of detecting CD63 on the surface of exosomes more specifically and at a higher sensitivity than Competitor A ELISA Kit and Competitor A ELISA Kit (high-sensitivity type).
PS Capture™ Exosome ELISA Kit (Streptavidin HRP)
-
Features
- Sensitive qualitative analysis (50 to 1000 folds more sensitive than WB in detection)
- Direct relative quantitation of extracellular vesicles in conditioned medium and body fluid specimens
- Application to detection systems using antibodies from various animal species and lectins
- Microanalysis with small amounts of extracellular vesicles
(only 1/10 to 1/1000 of the sample amount for WB)
-
Assay Principle
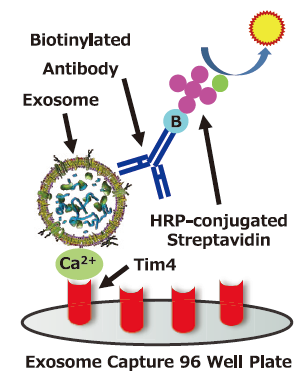
Exosome Capture 96 Well Plate which is pre-coated with protein that specifically binds to phosphatidylserine (PS) on the surface of extracellullar vesicles (EVs) capture EVs with Ca2+, Then biotinylated antibody for the surface marker protein of EVs is used as a primary detection and HRP-conjugated streptavidin is used as a secondary detection.
Application Data
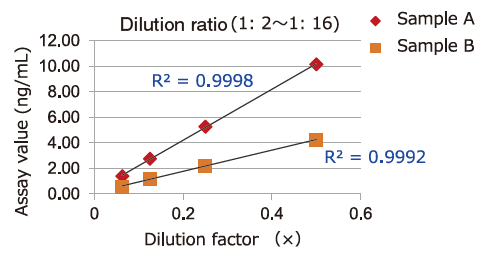
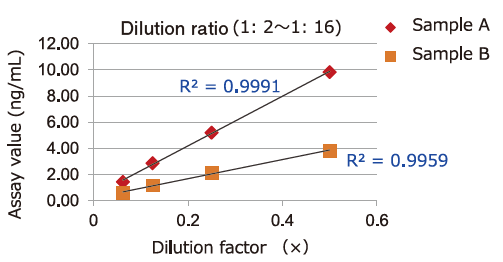
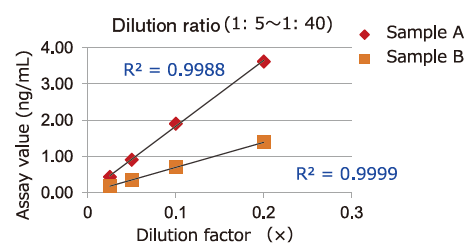
Dilution Linearity of Cell Culture Supernatant and Blood Samples
Dilution linearity of four specimens, ① human serum diluent ② human heparin plasma diluent ③ human EDTA plasma diluent (2 specimens, 4-step serial dilution each) and ④ COLO201 cells cell culture supernatant diluent (4-step serial dilution), was assessed by measuring the concentration of exosomes (CD63 detection) with standard curve obtained using extracellular vesicles purified from cell culture supernatant of COLO201 cells.
-
①Serum
-
②Heparin plasma
-
③EDTA plasma
-
④COLO201 cell culture supernatant
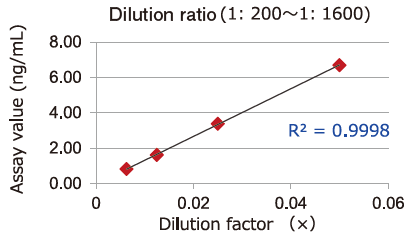
Samples in serum and heparinized plasma have given favorable linearity when diluted 2 folds or more.
Even samples in EDTA-treated plasma will give favorable linearity when diluted 5 folds or more.
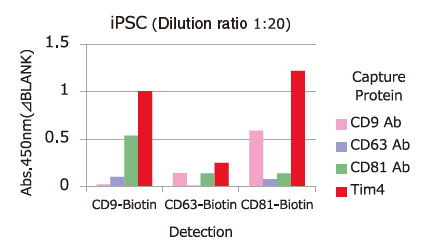
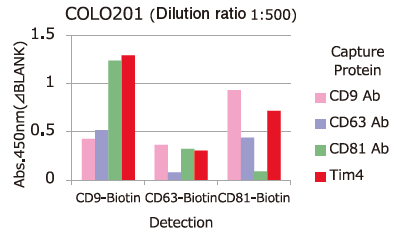
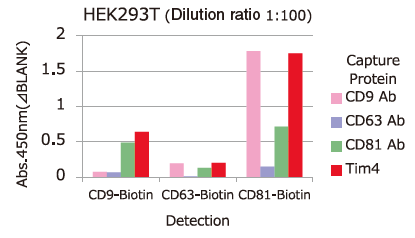
Comparison of Capture Ability between PS Affinity and Antibody
Microplate wells, pre-coated with anti-CD antibodies (CD9, CD63, CD81) and PS Affinity (Tim4), were incubated with pretreated ※ 1 cell culture supernatants of iPSC, COLO201 and HEK293T cells. Captured exosomes on the plate were detected using biotinylated anti-CD antibodies (CD9, CD63, CD81).
※ 1 Pretreatment condition: 10,000 x g, 30min.
Almost all of the exosomes derived from various cell lines were efficiently captured by Tim4 compared with anti-CD9, 63, 81 antibodies.
Spike and Recovery Assay with Blood Samples【EpCAM】
Pretreated※2 pooled normal human serum, EDTA-treated plasma, and heparinized plasma were spiked with exosomes isolated and purified from COLO201 cell conditioned medium using MagCapture™ Exosome Isolation Kit PS (Code No. 293-77601) at various concentrations. The spiked exosomes in a sample were detected using biotin-labeled anti-EpCAM※3 antibody (MBL), and the recovery rate of exosomes from the sample was calculated based on the standard curve prepared using exosomes purified from COLO201 cell conditioned medium.
※ 2 Pretreatment condition: 10,000 x g, 30min. ※ 3 EpCAM: Epithelial cell adhesion molecule
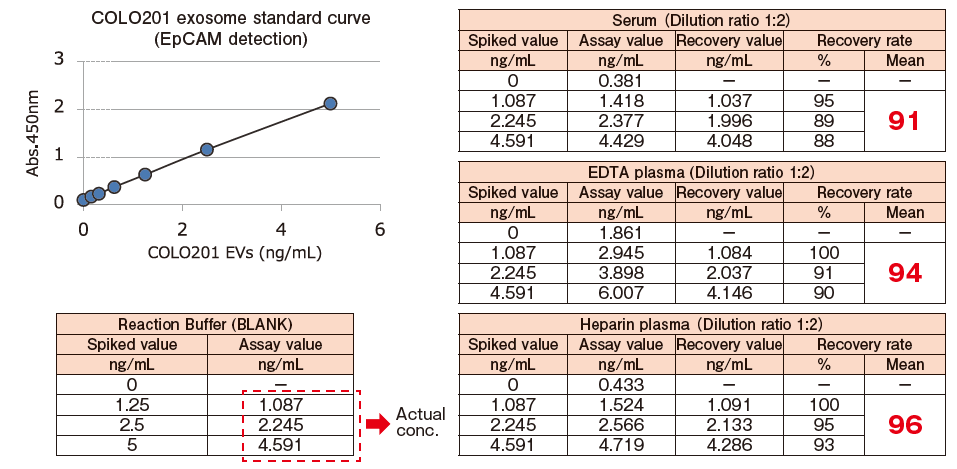
The spiked recovery rates based on the detection of EpCAM fell within a range of 100%±10%, showing favorable recovery performance.
Test of Residual Extracellular Vesicles in EV-depleted FBS
Extracellular vesicles in untreated FBS, commercial EV-depleted FBS and ultracentrifugation (UC)-treated FBS ※ 4 were detected using Biotinylated anti-CD9 antibody ※ 5.
Capture: Tim4 / Detection: Biotinylated anti-CD9 antibody
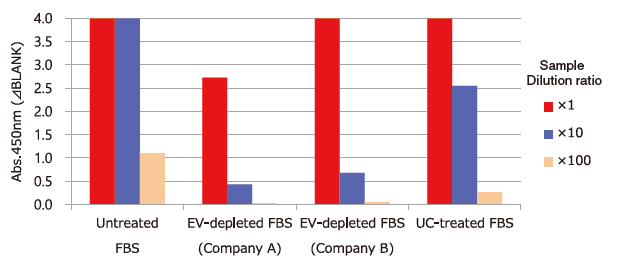
※ 4 Ultracentrifugation condition: 160,000 x g, 16h
※ 5 Anti-CD9 antibody (Code No. 014-27763) was biotinylated using Biotin Labeling Kit-SH (Code No. 348-90941).
PS Affinity ELISA System enables test of residual extracellular vesicles in EV-depleted FBS.
Sugar Chain Analysis by Sandwich ELISA with rBC2LCN Lectin and Tim4
Exosomes in five-fold dilution of pretreated※6 cell culture supernatants of iPS cells were detected using Biotinylated anti-CD63 antibody (Code No. 019-27713) and Biotinylated rBC2LCN lectin※7.
Capture: Tim4 / Detection: Biotin labelled compounds
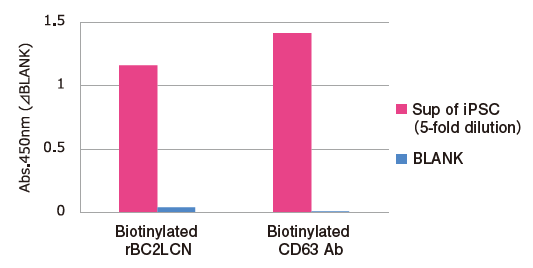
※ 6 Pretreatment condition: 10,000 x g, 30min.
※ 7 rBC2LCN binds specifically to Fucα1-2Galβ 1-3GalNAc (GlcNAc) and is known as a undifferentiated marker of iPS and ES cells. rBC2LCN (Code No. 029-18061) was biotinylated using EZ-Link™ Sulfo-NHS-LC-LC-Biotin (ThermoFisher).
Reference: S. Saito, et al., Sci. Rep., 8, 3997 (2018)
PS Affinity ELISA is applicable for sugar chain analysis on exosomes with a various combination of lectins.
Comparison of recovery efficiency with Polymer Precipitation
From 1 mL of pretreated ※ 8 COLO201 cell conditioned medium, exosomes were isolated and purified by PS affinity method or polymer precipitation method (competiter A) and then measured for signals of CD63, an exosome marker, using PS Capture™ Exosome ELISA Kit (streptavidin HRP). From absorbance of each sample, the amount of residual exosomes in a post-purification sample and the recovery rate of exosomes were determined for comparison.
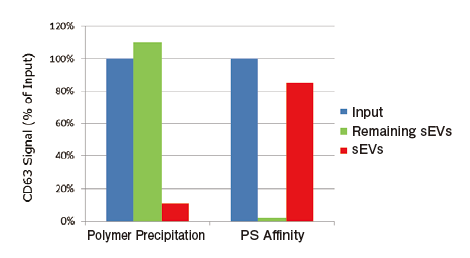
Calculation of recovery efficiency
Input: Measured value of 100-fold diluted conditioned medium※Ⅰ
Remaining sEVs: Measured value of post-purification 100-fold diluted conditioned medium ※Ⅰ
Recovered sEVs: Measured value of 1000-fold diluted purified sample ※Ⅱ
※Ⅰ Measured value of 100-fold diluted sample has been confirmed to fall within the measurement range. In the preliminary investigation, a dilution ratio appropriate for ELISA is recommended to be calculated for each sample
※Ⅱ Exosome samples purified with this kit and polymer reagents are theoretically concentrated 10 folds. (e.g. 1 mL of input → 100μL of purified exosomes) Value obtained by multiplying the concentration ratio by the dilution ratio for ELISA corresponds to integrated dilution ratio of the purified exosome for ELISA
The ELISA system allowing highly sensitive detection of exosom es will ease efficiency comparison among exosome recovery methods and validation of the method.